Green energy revolution: New breakthrough in hydrogen fuel cell technology
In a groundbreaking advancement, collaborative research has significantly reduced hydrogen fuel cell costs by replacing expensive platinum metals with silver catalysts, which are cheaper and more abundant. This marks a crucial stride toward making green energy storage both affordable and efficient.
As the world shifts to renewable energy, a key challenge emerges: effective energy storage during non-productive periods of solar and wind power. Hydrogen fuel cells, a leading contender, received a substantial boost from fundamental research conducted by the US Department of Energy’s SLAC National Accelerator Laboratory, Stanford University, and the Toyota Research Institute (TRI).

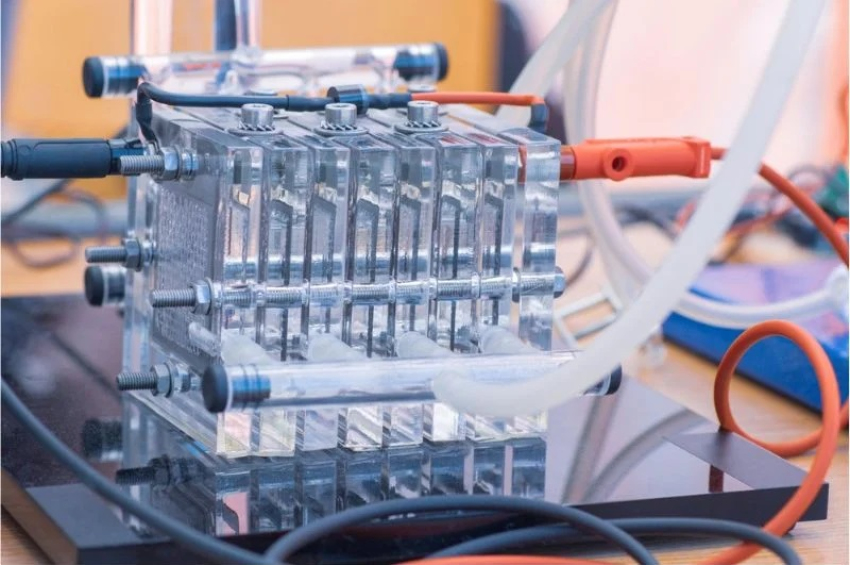
Platinum is used as a catalyst in proton-exchange membrane fuel cells. Credit: EEPower
This research translated into a practical fuel cell device through collaboration between Stanford and Technion Institute of Technology in Israel, with the results being published in the journal Nature Energy.
Michaela Burke Stevens, an associate scientist with SLAC and Stanford University’s SUNCAT Center, an a co-author of this research, notes that hydrogen fuel cells has a great potential for energy storage and conversion, emphasizing their role as an alternative to gasoline.
More to read:
How to store energy in red bricks with nanotech
However, the operational cost of hydrogen fuel cells remains a challenge and manufacturers in the automotive sector, for example, would not accept this solution.
In addition to high costs, using platinum also leads to potential degradation and raises environmental concerns.
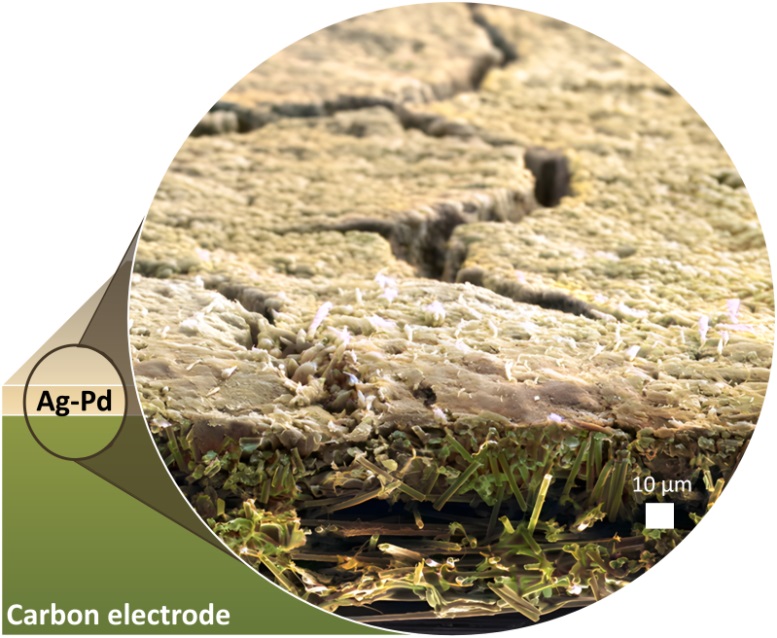
A silver-palladium thin film deposited on a porous carbon electrode. Credit: ScienceDaily
The obstacle lies in the catalyst, typically packed with expensive platinum group metals (PGM), essential for the chemical reaction that powers the system. Burke Stevens and her colleagues sought ways to make the catalyst more affordable, a daunting challenge requiring a fundamental change in the fuel cell’s chemistry.
More to read:
[videos] Why linear aerospike engine failed adoption in aerospace industry
This time, the researchers achieved cost balance by partially replacing PGMs with a more economical alternative - silver. The key innovation was simplifying the chemical process for applying the catalyst to the cell’s electrodes.
Traditionally, scientists mixed the catalyst into a liquid and spread it onto the electrode mesh. However, these recipes faced challenges in real-world applications due to variations in lab environments and tools. To address this, the SLAC team used a vacuum chamber for controlled depositions of the new catalyst onto electrodes.
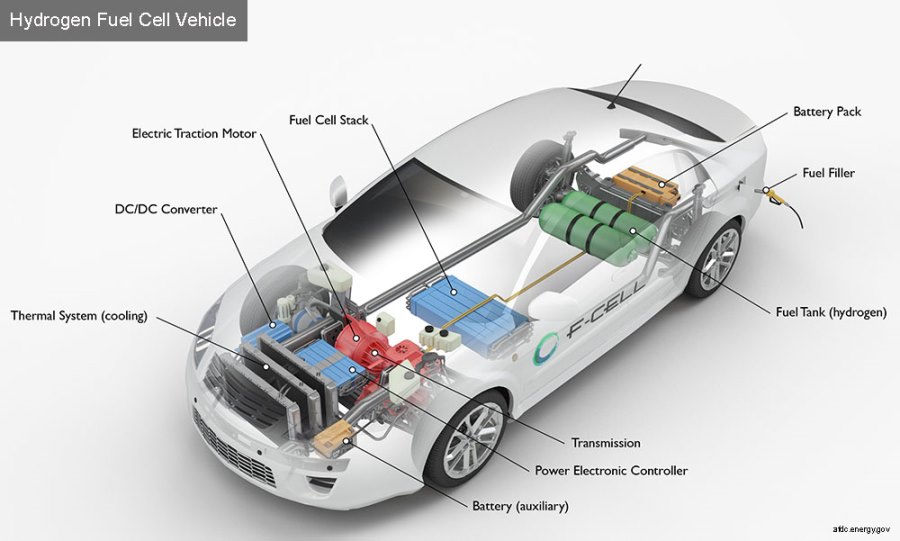
How the hydrogen fuel cells work in a vehicle. Credit: US Energy Department
Collaborating with Technion, the researchers demonstrated the method's practical application in a fuel cell setting. Substituting cheaper silver for some PGMs resulted in an equally effective fuel cell with a significantly lower cost.
Now, equipped with a proven method, they can explore more ambitious ideas, including potentially creating PGM-free fuel cells. One idea is to determine whether fuel cells are potent enough in heavy-duty transportation and clean energy storage.
This research received funding from the DOE’s Office of Science through the SUNCAT Center for Interface Science and Catalysis, a SLAC-Stanford joint institute, and the Toyota Research Institute.
***
NewsCafe is committed to quality journalism and relies on advertising to stay afloat. You can also buy us a coffee via PayPal: office[at]rudeana.com. Any help is welcome.